Hepatocellular carcinoma (HCC) is one of the most prevalent liver cancers worldwide, particularly affecting patients with underlying cirrhosis. Standard treatment includes surgical resection, liver transplantation, or percutaneous radiofrequency ablation (RFA).
However, in cases where percutaneous RFA is technically unfeasible—due to difficult tumor locations or associated procedural risks—alternative modalities are needed.
This case series explores the feasibility, safety, and efficacy of EUS-guided RFA, a minimally invasive method performed under Endoscopic Ultrasound (EUS) guidance for tumor ablation.
To evaluate clinical outcomes and technical success of EUS-guided RFA for HCC lesions located in challenging or anatomically sensitive liver segments in cirrhotic patients unfit for conventional procedures.
Diagnosed cases of Hepatocellular Carcinoma (HCC)
Tumor size ≤ 3 cm
Liver cirrhosis: either compensated or decompensated
Technically difficult tumor locations for percutaneous RFA
Not eligible or willing for surgical resection or liver transplantation
Age range: 45 to 71 years
Etiology of liver disease: Hepatitis B, NASH-related cirrhosis, Alcoholic liver disease
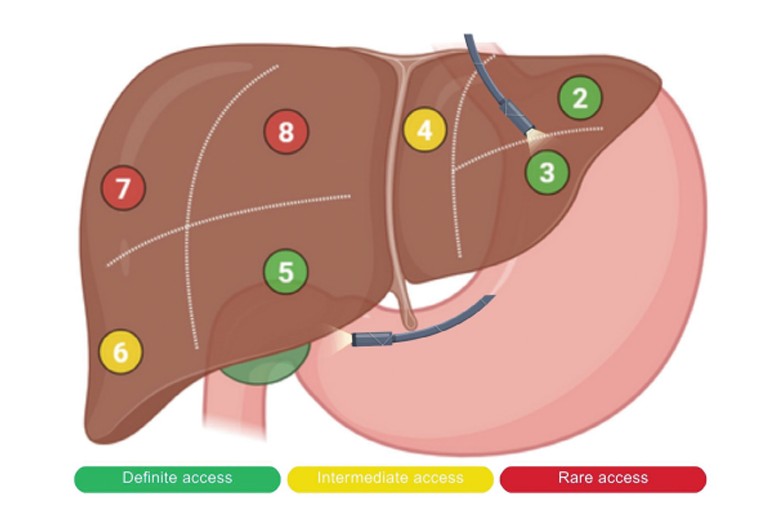
Tumor locations: Segments II, III, VI, VII, and VIII of the liver
Child-Pugh score: A (2 patients), B (2), C (1)
Linear-array Echoendoscope (GF-UCT180; Olympus Medical Systems)
19G Monopolar RFA needle (EUSRA, STARmed)
VIVA COMBO RF Generator (STARmed) with saline-cooled technology
Patients were sedated using conscious sedation or general anesthesia
Lesion identification via EUS using real-time Doppler and B-mode
The RFA needle was introduced transgastrically or transduodenally, based on tumor position
Ablation settings: 30W power, 15–20 seconds per application
Each tumor was treated with multiple passes (2–3 ablation cycles) to ensure complete coverage
The procedure was performed in a day-care setting or with overnight observation
Complete response in 4 out of 5 patients as confirmed by triphasic CT scans
Partial response in 1 patient (underwent a successful second EUS-RFA session)
Significant reduction in AFP (Alpha-Fetoprotein) levels
Pre-treatment AFP median: 158 ng/mL
Post-treatment AFP median: 23 ng/mL
No major complications reported
No evidence of bleeding, infection, or injury to adjacent structures
Mild post-procedure abdominal discomfort in 2 patients (resolved within 24 hours)
Offers precise targeting of lesions in challenging liver segments (e.g., Segment II, VIII)
Safer than percutaneous RFA in patients with ascites, coagulopathy, or proximity to vital structures
Real-time monitoring and thermal imaging enhance safety and effectiveness
Percutaneous RFA carries higher risk in posterior or left lobe tumors, obese patients, and tumors near vessels/organs
EUS-RFA bypasses anatomical limitations via transgastric or transduodenal access
This case series demonstrates that EUS-guided RFA is a safe, effective, and repeatable method for the treatment of small hepatocellular carcinoma lesions in cirrhotic patients, especially where standard RFA is not feasible.
It highlights the technical feasibility, clinical safety, and radiological success of the procedure—paving the way for broader adoption of EUS-RFA in hepatobiliary oncology.
✅ EUS-RFA achieved complete tumor ablation in 80% of patients after a single session
✅ No major complications observed
✅ Ideal for tumors in segments II, III, VII, and VIII where percutaneous access is limited
✅ Supports minimally invasive management in high-risk liver cancer patients
Harwani Y, Butala S, Shukla V, Patel A, Dogra Jani S.
Treatment of Hepatocellular Carcinoma Using Endoscopic Ultrasound‐guided Radiofrequency Ablation: A Case Series.
DEN Open. 2025;6:e70171. DOI: 10.1002/deo2.70171